A pharmaceutical liquid packed in glass container failed in stability studies as it found appearance of opalescence, Foreign particles and suspended particles. Not all the samples of the same batch found the abnormality. Our team had a detailed discussion with clients and concluded to start with preliminary investigation for delamination studies.
Delamination studies were initiated once the failed sample showed abnormal Zeta potential values when compared with controlled samples. The failed liquid samples were initially filtered using 0.2 micron filters. Isolated particulates were examined microscopically and elemental analysis was performed to identify the source of the particles. Empty glass container were further processed for qualitative evaluation on inner surface for Delaminated area and Roughening.
We have developed In-house standard protocols for Delamination propensity pre-screening studies. Glass sampling technique is very crucial and important aspect for delamination studies for which Nishka research have developed exemplary protocols, which are by leading pharmaceutical companies as they are showing promising results. SEM confirmed the inner surface pits on the wall near to bottom. Particles retained on filter paper confirmed glass particles as the elemental analysis with EDS are matching with glass composition. We confirmed that delamination is taking place, during the product development stage and helped our client in forecasting the delamination problem. Prototype results are presented below (Figure-1 & 2)


Exemplary comparison of controlled and delamination sample

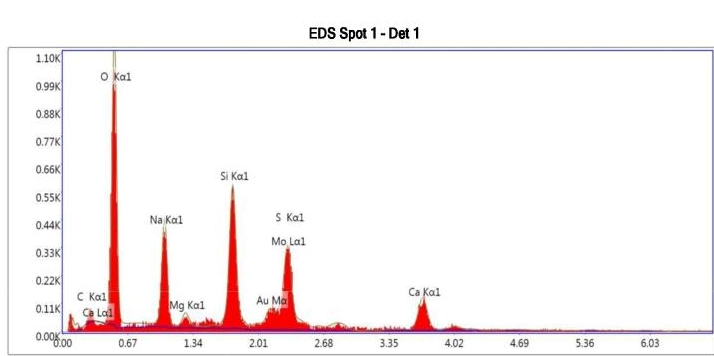
Confirmation of delaminated glass particles using EDS
Need help or Have a question?
Nishka Research Pvt. Ltd.
Regus Business Center,
4th floor, Gumidelli Commercial Complex,
1-10-39 to 44, Old Airport Road, Begumpet,
Hyderabad-500016
India
Phone : +91 40 29303155
Mobile : +91 7842798518
Left us a star review